Learning Outcomes
i. Define oxidizing and reducing agents, recognizing their fundamental roles in electron transfer processes.
ii. Analyze the characteristics of oxidizing and reducing agents, including their tendencies to gain or lose electrons.
iii. Identify oxidizing and reducing agents in redox reactions based on their electronegativity differences.
iv. Apply the concept of oxidation states to determine the identities of oxidizing and reducing agents in chemical reactions.
v. Explain the significance of oxidizing and reducing agents in various chemical processes, including combustion, corrosion, and photosynthesis.
Introduction
In the intricate world of chemistry, redox reactions, the silent symphony of electron exchange, govern a vast array of transformations. At the heart of this electron dance lie oxidizing and reducing agents, the key players that drive chemical change. This lesson will delve into the roles and characteristics of these essential substances, empowering you to identify them in redox reactions and appreciate their significance in shaping our world.
i. Oxidizing Agents: Masters of Electron Acquisition
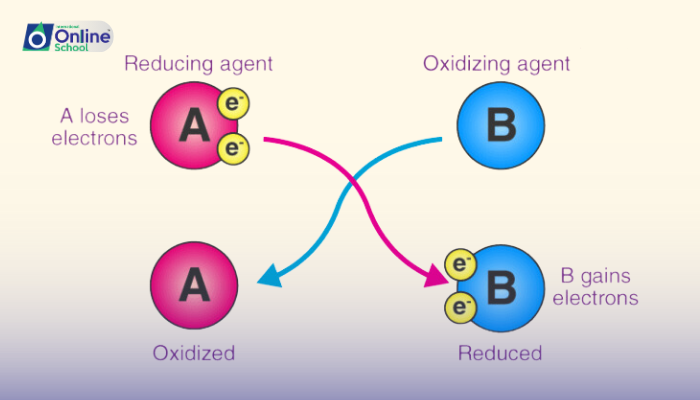
Oxidizing agents, the electron acceptors in redox reactions, are the avid seekers of electrons. They possess a high electronegativity, enabling them to attract and hold electrons firmly. During redox reactions, oxidizing agents undergo reduction, gaining electrons and decreasing their oxidation states.
Characteristics of Oxidizing Agents:
High electronegativity: Oxidizing agents readily attract electrons due to their strong affinity for electrons.
Electron acceptors: Oxidizing agents willingly accept electrons from other substances, undergoing reduction.
Reducing agents: Oxidizing agents cause other substances to lose electrons, making them reducing agents.
ii. Reducing Agents: Masters of Electron Donation
Reducing agents, the electron donors in redox reactions, are the generous providers of electrons. They possess a relatively low electronegativity, making them prone to losing electrons. During redox reactions, reducing agents undergo oxidation, losing electrons and increasing their oxidation states.
Characteristics of Reducing Agents:
Low electronegativity: Reducing agents readily lose electrons due to their weak affinity for electrons.
Electron donors: Reducing agents willingly surrender electrons to other substances, undergoing oxidation.
Oxidizing agents: Reducing agents cause other substances to gain electrons, making them oxidizing agents.
iii. Identifying Oxidizing and Reducing Agents: A Matter of Oxidation States
In the intricate dance of redox reactions, the identities of oxidizing and reducing agents can be determined by analyzing the changes in oxidation states of the species involved. The species that loses electrons and increases its oxidation state undergoes oxidation and acts as the reducing agent, while the species that gains electrons and decreases its oxidation state undergoes reduction and acts as the oxidizing agent.
iv. Electronegativity: A Guiding Principle
Electronegativity, the measure of an atom's ability to attract electrons, provides a valuable clue in predicting the tendency of substances to act as oxidizing or reducing agents. Generally, substances with higher electronegativities tend to act as oxidizing agents, while substances with lower electronegativities tend to act as reducing agents.
v. The Significance of Oxidizing and Reducing Agents: Shaping Our World
Oxidizing and reducing agents play pivotal roles in a wide range of chemical processes, shaping our world from the energy we consume to the life we sustain. Combustion reactions, the burning of fuels, rely on the electron-accepting nature of oxidizing agents to release energy. Corrosion, the deterioration of metals, involves the reduction of metals by oxidizing agents, such as oxygen or moisture. And photosynthesis, the process by which plants produce energy, utilizes the electron-donating capacity of reducing agents, such as water, to convert carbon dioxide into life-sustaining molecules.
Oxidizing and reducing agents, the essential partners in redox reactions, orchestrate a myriad of chemical transformations, shaping our world from the energy we harness to the life we nurture. Understanding these fundamental substances and their roles in redox reactions provides a powerful tool for analyzing chemical processes, predicting outcomes, and appreciating the intricate dance of electrons in the grand symphony of chemistry.